|
What
is the focus of this module? What is the compelling problem that students will face in this module? Working in teams, students will review the many issues that surround ozone depletion. They will evaluate the current status of the Montreal Protocol, considering the interrelationships of earth's spheres, including the anthrosphere. Students must investigate how an event in one sphere may affect a second sphere, which may, in turn, affect a third sphere. Students should determine if there is a need for revision of the Montreal Protocol and, if so, in what areas. Students should be prepared to defend the advice they give. What
topics/tasks/issues will students encounter/raise as they work through
this module? The issue of developing nations and CFC reduction should be addressed. These nations were given extra time to come into compliance. The pressing issue now is how much of the cost of compliance should be born by the developed countries. There are social and humanitarian aspects that should be considered. For example, developing countries may say, "We are not responsible, why should we have to pay? Besides, we have more important things to worry about--like feeding our people." What
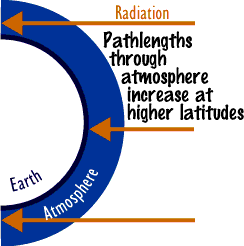
is the role of remote sensing in this module? Activity 2 is somewhat complex and probably beyond the capability of the average student. Most students will want guidance. On the first day of spring, sun angle largely determines the amount of radiation incident at the top of the atmosphere. At the equator, the sun is directly overhead. At 30° latitude, the sun will make an angle of 30° with the vertical solar zenith angle, so the top of the atmosphere receives about 86.6% of the radiation received at the equator (cos 30° x total radiation). At 60° latitude, only 50% of the amount received at the equator is received . Likewise, the path length the radiation takes increases with latitude. At 30°, it is 15% more than that at the equator (sec 30° x path length at equator). This is a multiplier effect as far as attenuation of radiation is concerned. At 60° latitude, the path length is doubled, effectively doubling the effect of ozone at 60° when compared with the equator. For example, suppose on the first day of spring, ozone concentrations are 220 Dobson Units at the equator and 440 Dobson Units at 60° latitude. At 60°, solar radiation at the top of the atmosphere is half what it is at the equator. At 60°, path length essentially doubles the effectiveness of the 440 Dobson Units. Assuming each one percent increase in ozone reduces uvb radiation by two percent, the ozone at 60° N would reduce uvb eight times as effectively as the ozone at the equator. The result is that uvb radiation reaching the ground at 60° is about 1/16 of that at the equator on the first day of spring. The problem changes at different times of the year. In the summer, because of longer days and smaller zenith sun angles, as one approaches the pole, radiation at the top of the atmosphere is fairly uniform from the tropic of cancer to about 60° latitude. On the other hand, radiation decreases very rapidly in the direction of the pole during the winter. Students may ask the purpose of activity 3. In activity 3, students should see some relation between O3, HNO3, and CLONO2 concentrations. As HNO3 is taken out of the atmosphere in the cold Antarctic winters and condenses into its liquid form in PSCs, it is no longer available to react with ClO. ClO is an unstable form of chlorine important in ozone reduction. When HNO3 undergoes photolysis in the spring, it is capable of reacting with Cl and ClO, forming the much more stable form of chlorine, ClONO2. Early in the spring, much chlorine is available to react with ozone, but as the HNO3 dissociates, increasing amounts of ClONO2 form. So, after about three weeks the ozone hole begins to close. Because of atmospheric circulations, there are spatial and temporal variabilities, so you would not expect to see an exact correlation at every point plotted. Preparation Checklist--have you thought of everything? |
|
Grade
Level: 10-12
|
|
Resources
for this module * On this calculator, for the English system, temperatures are given in °R, so 459 must be subtracted to obtain °F. Likewise, for the metric system subtract 273 from °K to obtain °C. |
HTML code by Chris
Kreger
Maintained by ETE
Team
Some images © 2004 www.clipart.com
Privacy Statement and Copyright © 1997-2004 by Wheeling Jesuit University/NASA-supported Classroom of the Future. All rights reserved.
Center for Educational Technologies, Circuit Board/Apple graphic logo, and COTF Classroom of the Future logo are registered trademarks of Wheeling Jesuit University.