
Water
Quality Assessment: Chemical: Conductivity and Density
Conductivity is
the ability of water to carry an electrical current. It indicates the
physical presence of dissolved chemicals in the water. For example, when
sodium chloride (NaCl, table salt) dissolves in water, it dissociates
into Na+ and Cl- ions. The movement of these ions conducts electricity
through the water. The dissociation of naturally-occurring, inorganic
compounds is the main source of ions in stream water. Conductivity can
also increase as a result of heavy metal ions released from pollutants
such as acid mine drainage.
To "see" the electrical current being carried by Na+ and Cl- ions, try the following activity. First, connect the positive terminal of a battery to an electrode. Place the electrode in a glass of deionized water. Next, connect the negative terminal of the battery to a light bulb, then connect the light bulb to an electrode. Place the electrode in a glass of deionized water (see animation below). Notice that the light bulb does not glow. Add some salt (NaCl) to the deionized water (see animation below). Notice that the light bulb starts to glow. That is because the Na+ and Cl- ions complete the circuit between the positive and negative electrodes. They enable the electrical current to flow through the circuit.
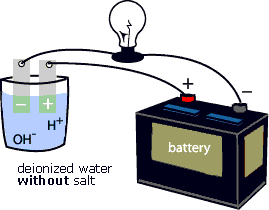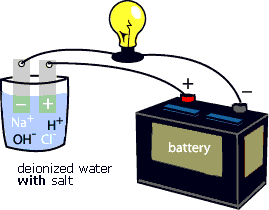
Like conductivity, water density is an indicator of the physical presence of chemicals in water. The density of water is related to salt content and water temperature. This is a very useful parameter to measure because the salinity of a body of water is one of the main factors determining what organisms will be found there.
Overview ..|.. Biological Assessment ..|.. Chemical Assessment ..|.. Physical Assessment.
pH
/ Alkalinity / Hardness
/ Nitrates. Nitrites, and Ammonia / Ortho-
and Total Phosphate / Dissolved Oxygen and
Biochemical Oxygen Demand / Fecal Coliform
/ Conductivity and Density
Glossary .|.
Related Links
.|..
References
..|..
PBL Model
.|
Home ..|.. Teacher Pages ..|.. Modules & Activities
HTML code by Chris Kreger
Maintained by ETE Team
Last updated November 10, 2004
Some images © 2004 www.clipart.com
Privacy Statement and Copyright © 1997-2004 by Wheeling Jesuit University/NASA-supported Classroom of the Future. All rights reserved.
Center for Educational Technologies, Circuit Board/Apple graphic logo, and COTF Classroom of the Future logo are registered trademarks of Wheeling Jesuit University.
