
Water
Quality Assessment: Chemical: Dissolved Oxygen and Biochemical Oxygen
Demand
Oxygen is essential
for the survival of nearly every living thing — even those living
in water. The two main sources of dissolved oxygen in stream water are
the atmosphere and aquatic plants. Atmospheric oxygen is mixed into stream
water as waves crash along the riffles.
Aquatic plants introduce oxygen into stream water as a byproduct of photosynthesis.
The amount of oxygen that can dissolve in water is limited by physical
conditions such as temperature and atmospheric
pressure.
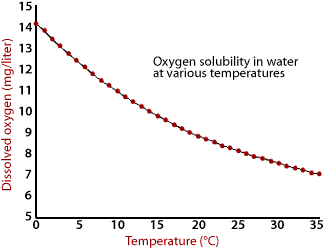
Data
courtesy of Dr. Ben Stout.
The above graph shows the maximum amount of oxygen that can be dissolved in water at various temperatures. Assuming a constant atmospheric pressure, water of low temperatures can hold more oxygen than water of high temperatures.
One unit of measure of dissolved oxygen in water is parts per million (ppm), which is the number of oxygen (O2) molecules per million total molecules in a sample. Calculating the percent saturation is another way to analyze dissolved oxygen levels. Percent saturation is the measured dissolved oxygen level divided by the greatest amount of oxygen that the water can hold at that particular temperature and atmospheric pressure, then multiplied by 100.
Fish growth and activity usually require 5-6 ppm of dissolved oxygen. Dissolved oxygen levels below 3 ppm are stressful to most aquatic organisms. Levels below 2 ppm will not support fish at all.
Low dissolved oxygen levels can be the result of elevated temperature and thus the inability of the water to hold the available oxygen. Low dissolved oxygen levels can also indicate an excessive demand on the oxygen in the system. Some pollutants such as acid mine drainage produce direct chemical demands on oxygen in the water for certain oxidation-reduction reactions. Other pollutants such as sewage or agricultural runoff result in the build up of organic matter and the consumption of dissolved oxygen by microbial decomposers as they break down the organic matter.
The measure of the amount of oxygen used by aerobic bacteria during decomposition is called biochemical oxygen demand (BOD). Biochemical oxygen demand can be determined by collecting two water samples and immediately measuring the dissolved oxygen concentration in one of them--Sample 1. The second sample--Sample 2--is incubated at room temperature for 5 days. The incubation bottle should be in complete darkness to prevent the production of more dissolved oxygen within the sample through the process of photosynthesis. Bacteria in the incubated sample will metabolize normally, consuming dissolved oxygen in the process. After the 5 day incubation period, the dissolved oxygen concentration in Sample 2 is determined. To calculate BOD, subtract the amount of dissolved oxygen in Sample 2 from the amount of dissolved oxygen in Sample 1. This is the amount of oxygen that has been used, or demanded, by microbes during the 5-day incubation period. Unpolluted natural waters will have a BOD of 5 mg/L or less.
Overview ..|.. Biological Assessment ..|.. Chemical Assessment ..|.. Physical Assessment.
pH
/ Alkalinity / Hardness
/ Nitrates. Nitrites, and Ammonia / Ortho-
and Total Phosphate / Dissolved Oxygen and Biochemical Oxygen Demand
/ Fecal Coliform / Conductivity
and Density
Glossary .|.
Related Links
.|..
References
..|..
PBL Model
.|
Home ..|.. Teacher Pages ..|.. Modules & Activities
HTML code by Chris Kreger
Maintained by ETE Team
Last updated November 10, 2004
Some images © 2004 www.clipart.com
Privacy Statement and Copyright © 1997-2004 by Wheeling Jesuit University/NASA-supported Classroom of the Future. All rights reserved.
Center for Educational Technologies, Circuit Board/Apple graphic logo, and COTF Classroom of the Future logo are registered trademarks of Wheeling Jesuit University.
