
Carbon
Dioxide: Overview
Compounds that
contain the element carbon are referred to as "organic." These
compounds are very important. They are present in all living things. The
carbon element is continually moving among the earth's lithosphere,
hydrosphere, biosphere, and atmosphere in various forms--as carbon dioxide
(CO2), sugars or carbohydrates (CnH2nOn), and and calcium carbonate
(CaCO3), to name just a few. The movement of carbon
among the earth's spheres, as diagrammed below, is known as the carbon
cycle.
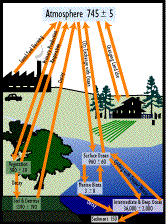
Green plants play a very important
role in the carbon cycle. They absorb carbon dioxide (CO2) from the atmosphere
and produce carbon-containing sugars. This process is called photosynthesis.
There are two main steps in photosynthesis. First, plants trap the sun's
light energy in a compound called chlorophyll. This energy is converted to
a chemical form called adenosine triphosphate (ATP). In the second step,
plants use the energy from ATP to produce sugar (C6H1206). The process of photosynthesis
requires water (H2O). It also produces water, as well as oxygen
(O2). The
net chemical reaction for the process of photosynthesis is:
6 CO2
+ 6 H2O + sunlight ------> C6H12O6 + 6
O2.
Animals eat plants to obtain the energy trapped during photosynthesis. As the animals' bodies break down the carbohydrates in the plant tissue, CO2 is released to the atmosphere. This process is called respiration. The net chemical reaction for the process of respiration is the exact opposite of photosynthesis: 6 O2 + C6H12O6 ------> 6 H2O + 6 CO2. Plants, too, respire as they break down the organic molecules in themselves in order to release the stored energy. Plants and animals also release CO2 to the atmosphere when they decompose or decay. The chemical reaction for this process is the same as that for respiration.
When dead plants and animals slowly decay under high pressure and high temperatures, they may form
pools of energy known as fossil fuels. Fossil fuels include coal,
oil, and natural gas. People burn fossil fuels, as well as fresh
vegetation, to release the energy stored in them. The energy is used for
heat, operating automobiles, etc. The chemical process of burning
fuel--known as combustion--is the same as respiration and
decomposition:
6 O2 + C6H12O6 ------> 6
H2O + 6 CO2.
Since the Industrial Revolution, humans have burned increasingly greater amounts of fossil fuels in order to produce more energy. As the practice of burning fossil fuels grows, so does the amount of carbon dioxide emitted to the atmosphere. Historical data from ice cores and modern data collected from the Mauna Loa observatory in Hawaii support this. The graph below depicts a 30% increase in atmospheric concentrations of carbon dioxide (CO2) since 1860.
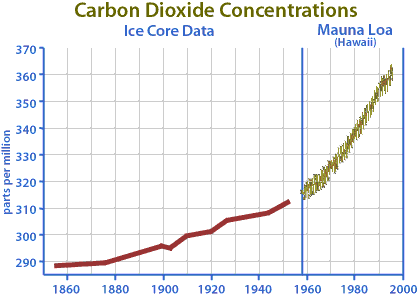
Image: Atmospheric concentrations of carbon dioxide from 1855 to 1996. Figure adapted from an image courtesy of the Whitehouse Initiative on Global Climate Change.
There is growing evidence that increases in atmospheric concentrations of CO2 may increase the rate of global climate change due to the greenhouse effect. This is because CO2 contributes to 55% of the greenhouse effect. Increases in atmospheric concentrations of CO2 may also have great impacts on plant growth by affecting rates of photosynthesis.
Overview
..|..
Temperature ..|..
Precipitation
..|..
Plants
Glossary ..|..
Related
Links ..|..
References
|..
PBL
Model
Home ..|.. Teacher Pages ..|.. Modules & Activities
HTML code by Chris
Kreger
Maintained by ETE
Team
Last updated November 10, 2004
Some images © 2004 www.clipart.com
Privacy Statement and Copyright © 1997-2004 by Wheeling Jesuit University/NASA-supported Classroom of the Future. All rights reserved.
Center for Educational Technologies, Circuit Board/Apple graphic logo, and COTF Classroom of the Future logo are registered trademarks of Wheeling Jesuit University.
